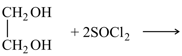Poly Halogen Compounds
Poly Halogen Compounds: Overview
This Topic covers sub-topics such as Chloroform, Carbon Tetrachloride, Vicinal Dihalides, Gem Dihalides, Preparation of Chloroform, Nomenclature of Freons, Uses of CH2Cl2, Preparation of CCl4 and, Reduction of Chloroform
Important Questions on Poly Halogen Compounds
The name of according to the nomenclature of freons is
Vicinal dihalides on treatment with zinc metal and loses a molecule of to form an _____.
Dibromoethane on treatment with zinc metal gives
What is the major product formed in the given reaction:
Describe the reaction of vicinal dihalides with zinc.
Methylene chloride is used as an aerosol spray propellant.
Methylene chloride is used as an _____ spray propellant.
Which of the following is not an application of methylene chloride.
Describe the uses of methylene chloride.
Chloroform on reaction with forms methane.
Mention the reagent used to convert chloroform to methylene chloride.
Describe the reduction process of chloroform.
Complete and balance the following equation:
Methane reacts with excess of _____ in presence of sunlight to undergo multiple substitution reactions to form chloromethane, dichloromethane, trichloromethane and tetrachloromethane respectively.
Methane on reaction with excess of chlorine in presence sunlight gives chloroform.
Describe the preparation of chloroform from methane.
Ethylene glycol reacts with excess of thionyl chloride to give a vicinal dihalide.
Ethylene glycol reacts with excess of to give
How are vicinal dihalides prepared from vicinal diols.
Complete the following reaction: